iProtin Assay Cartridge Kit employs SAW Biosensor chips with advanced automated protein coating technology, allowing users to detect a range of biomarkers in the human body within just three minutes. This rapid quantitative data provides doctors with immediate diagnostic insights and supports informed decision-making. Additionally, it enables individuals to monitor their health status effectively. This technology is currently in widespread use across major hospitals, clinics, and by home care physicians.
iProtin system enables multiple bio-markers’ tests in one common device.
- C-reactive protein assay cartridge kit (CE & TFDA approval)
- Lipoprotein(a) assay cartridge kit (CE, FDA listing & TFDA approval)
- Apolipoprotein B assay cartridge kit (FDA listing & TFDA approval)
- Albumin assay cartridge kit (FDA listing & TFDA approval)
- Microalbumin assay cartridge kit (Coming Soon)
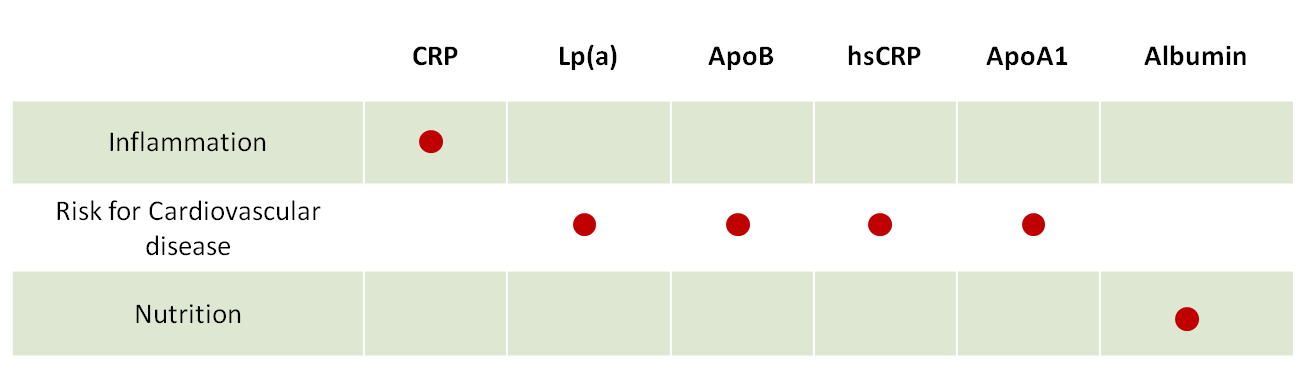
Detail of available biomarkers
- CRP - CRP an acute-phase protein, exhibits a non-specific surge in concentration during inflammatory processes, whether infectious or non-infectious. Given its association with pathological changes, CRP serves as a versatile indicator for diagnosing infections and inflammation. Furthermore, CRP is instrumental in monitoring post-treatment or post-surgery infection development in patients. The iProtin Reader adeptly captures these changes, enabling precise determination of CRP concentrations in whole blood.
- Lp(a) - assessment values emerge as a highly sensitive indicator in the development of coronary artery disease. When evaluating the comprehensive risk of atherosclerosis, Lp(a) measurements should be complemented by assessments of cholesterol, HDL, LDL, and TG. Furthermore, it is recommended to measure Lp(a) values in individuals with dyslipoproteinemia, diabetes mellitus, renal failure, cardiovascular or cerebrovascular disease, and early atherosclerosis. Given that Lp(a) levels are genetically determined and impervious to dietary or lifestyle modifications, screening for diagnosis becomes imperative. Identifying high-risk individuals through screening provides a crucial window for proactive measures to eliminate or manage other risk factors promptly.
- ApoB- ApoB, this product facilitates the quantitative detection of ApoB in human whole blood or fingerstick blood and must be utilized in conjunction with an iPrtotin reader. In clinical settings, the ApoB assessment holds significance as a diagnostic aid in determining the risk of cardiovascular disease. It serves as an indicator for evaluating the risk of coronary heart disease. Recent research findings indicate a negative correlation between Apo A1 and coronary heart disease, while ApoB exhibits a positive correlation. Patients with coronary heart disease typically manifest lower levels of Apo A1 and elevated levels of ApoB. Screening and diagnosing through this method offer a valuable opportunity to identify individuals at high risk, prompting timely intervention to eliminate or manage other risk factors associated with cardiovascular diseases.
Connected Paper
- Cardiovascular diseases evalution and tracking (hsCRP, Lp(a), Apo B, Apo A1)
- Inflammation, heart disease & bacterial/virus infections (CRP)
Operation videos
Operation Video - CRP
Operation Video - Lp(a)
Operation Video - ApoB
Operation Video - SARS-COV-2
Operational Guideline

Approval
FDA 

Manufacturer
.jpg)



